Innovative Medicine
Backed by the Power of AI
BioXcel Therapeutics, Inc. is a
biopharmaceutical company developing transformative medicines in neuroscience utilizing artificial intelligence.
Innovative Medicine
Backed by the
Power of AI
BioXcel Therapeutics, Inc., a clinical-stage biopharmaceutical company developing transformative medicines in neuroscience and immuno-oncology utilizing artificial intelligence, or AI, techniques.
Innovative Medicine
Backed by the Power of AI
BioXcel Therapeutics, Inc., a clinical-stage biopharmaceutical company developing transformative medicines in neuroscience and immuno-oncology utilizing artificial intelligence, or AI, techniques.
Our Approach to Drug Development
- Optimizes R&D economics
- Shortens development timelines
- Increases probability of success
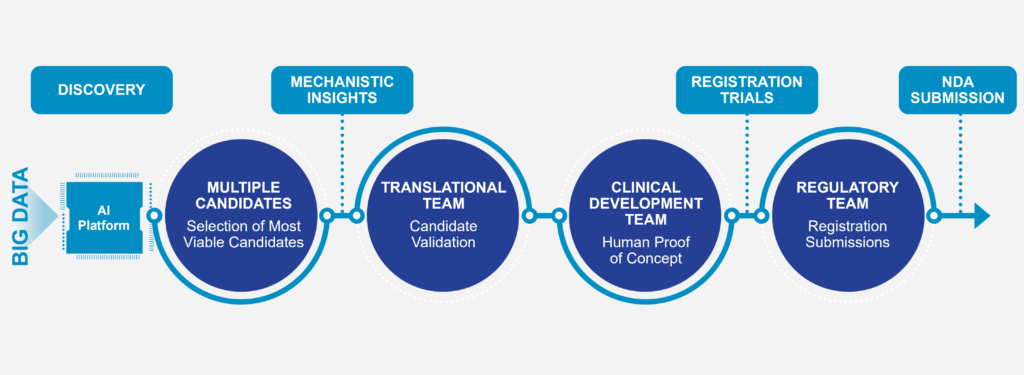
AI-Driven Transformative Medicines in Neuroscience
We utilize our unique and proprietary AI platform to identify, re-innovate, and develop potential new therapies

Apr 24, 2024
BioXcel Therapeutics Announces Late-Breaking Abstract on Preliminary Findings from Phase 2 Investigator-Sponsored Trial of BXCL701 and KEYTRUDA® in Metastatic Pancreatic Ductal Adenocarcinoma (PDAC) Selected for Presentation at 2024 ASCO Annual MeetingVIEW RELEASE
Apr 22, 2024
BioXcel Therapeutics Announces Plan for Evaluating BXCL501 in the At-Home Setting to Expand Its Market PotentialVIEW RELEASE
Apr 10, 2024
BioXcel Therapeutics Announces TRANQUILITY In-Care Pivotal Phase 3 Trial Plan With BXCL501 for Agitation Associated With Alzheimer’s DementiaVIEW RELEASE
Stock Information
BioXcel Therapeutics, Inc.
Apr 24, 2024 04:00 PM
MARKET/SYMBOL
Nasdaq:
BTAI
PRICE
2.71
CHANGE
0 (0%)