PRODUCT PIPELINE
The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established.
Our Neuroscience Clinical Pipeline
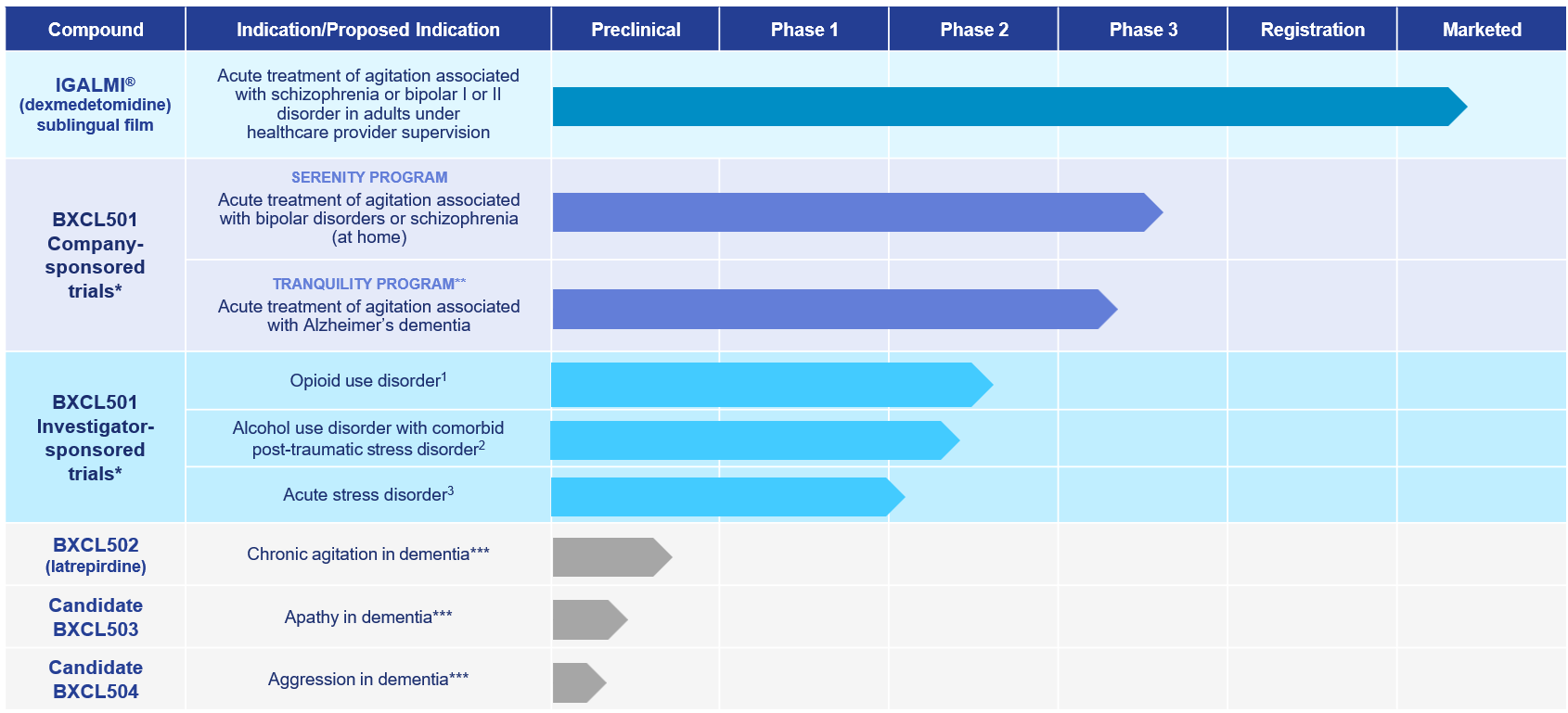
1 Collaborator: Columbia University
2 Collaborator: Yale University Medical School
3 Collaborator: University of North Carolina at Chapel Hill
*The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established
**Program paused subject to funding
***Development paused due to Strategic Reprioritization announced on Aug. 14, 2023
BXCL501

BXCL501 is our most advanced neuroscience clinical asset. In indications other than those approved by the FDA as IGALMI, BXCL501 is an investigational, proprietary, orally dissolving film formulation of dexmedetomidine, a selective alpha-2 receptor agonist targeting symptoms from stress-related behaviors such as agitation. Please see full Prescribing Information.
BioXcel Therapeutics believes BXCL501 potentially targets an important mediator of agitation and has observed anti-agitation results in multiple clinical trials across several neuropsychiatric disorders. BXCL501 is being evaluated for the potential acute treatment of agitation associated with dementia due to probable Alzheimer’s disease in the care setting, and for the acute treatment of agitation associated with bipolar I or II disorder or schizophrenia in the at-home setting. The safety and efficacy of BXCL501 for these investigational uses have not been established.
BXCL501 has been granted Breakthrough Therapy designation by the FDA for the acute treatment of agitation associated with dementia and Fast Track designation for the acute treatment of agitation associated with schizophrenia, bipolar disorders, and dementia.


